Effective, safe, economical and user-friendly
Cleanroom cleaning requires these factors. When one views the various purity classes (ISO and/or GMP) in the different industrial areas, the cleanrooms differ in the requirements and regulatory specifications.
In the technical industry, all types of particles can lead to interruptions, manufacturing stops or loss/reduction of quality. For this reason, in industries such as the semiconductor, microelectronics, optics, precision mechanics, nano technology, and aerospace technology, the avoidance of particulate contaminations is in the foreground. Even the cleaning equipment must not be a source of particles. For higher purity requirements, system trolleys made entirely of stainless steel can be used (GMP series) while in the lower purity classes, prescribed types of plastic can be used. The regulatory basis for the selection of the equipment for these cleanroom areas are, in particular, covered by the Standards ISO 14644-5 as well as VDI 2083, page 5.1.
In the industrial areas of medical technology, non-sterile medical products, foodstuffs and the cosmetics industry, a defined security in terms of particles and microbiological contamination is required; however, sterility is not mandatory. In order to ensure hygienic purity, the devices employed in the manufacturing area must not emit or distribute any contamination and must offer a high level of process reliability during day-to-day operations. Aside from the special hygiene regulations prevalent in the various sectors of industry, these cleanroom areas are also subject to additional requirements, in particular those noted in the standards ISO 14644-5 as well as VDI 2083, page 5.1. Depending on the demand in safety and quality, we recommend either the Science or the GMP series.
Through the pharmaceutical GMP guidelines, there are clear and specific regulations pertaining to the devices used in the manufacturing of medication. This ensures that any health risks and product spoiling are eliminated. The manufacturing equipment must be GMP conform, been processed using specialised materials, and must ensure a safe and reproducible wetting. Additional requirements – also for pharmaceutical cleanrooms – can be taken from the ISO 14644-5 and VDI 2083, page 5.1 standards.
The manufacturing of sterile medication and sterile medical products is, due to the sensitive nature of these products, additionally regulated through Annex 1 of the EU-GMP guidelines and the corresponding FDA guideline. In addition to the general GMP requirements and the requirements based on the standards 14644-5 and VDI 2083, page 5.1, the devices must be autoclavable and the processes must be validatable. All information must be validated through audits and certificates. The GMP series was once developed for the pharmaceutical industry – including the areas under sterile conditions – as well as for the industry sectors that had similar quality demands.
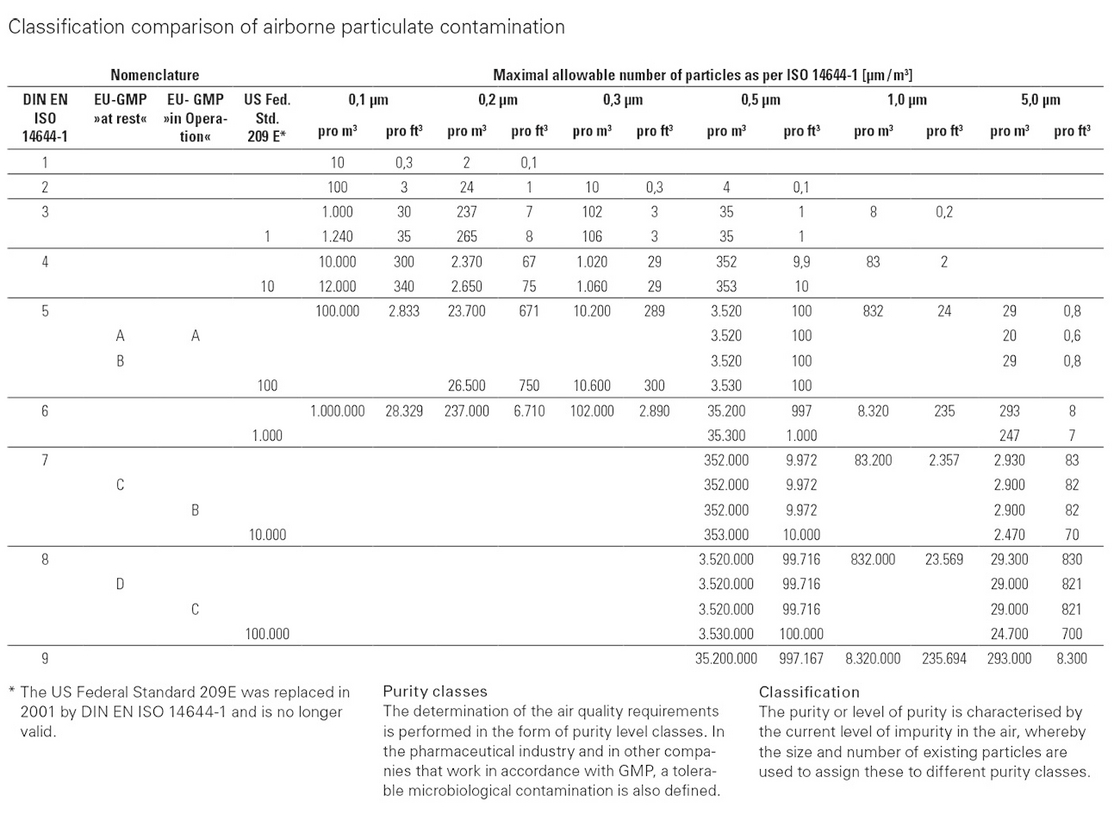
For you to have a good overview of the air purity classes, we've attached a valid table.
Indicator of cleanroom classification
For all on this webiste listed products for the cleanroom cleaning and disinfection there is this indicator. These mentioned classes are air purity classes. A specific allocation of consumable materials to these classes can only have an orientation character. Although a large number of measured parameters and independent studies exist, the suitability for the respective production process has to be assessed besides by the user.

Rules and regulations
Focus on the details and the goal: Understanding regulations, adhering to them, and integrating them into the best systems.
more